
STOP-MS is a multi-arm, multi-stage (MAMS) clinical trial of anti-viral agents in the treatment of progressive MS. It is a Phase 3 clinical trial.
The drugs used in Stage 1 and Stage 2 of the trial will be tested against a single placebo group.
The treatments tested will be repurposed treatments, meaning they are already approved for use in Australia for other conditions, and their safety profile is well-described. These treatments will largely be focused on suppressing the Epstein Barr virus (EBV), in the hope that this will slow disease progression. To learn more about EBV in MS, visit the EBV in MS webpage.
New arms could potentially be added to STOP-MS in the future to test other new and promising drugs.
STOP-MS will be conducted at multiple sites throughout Australia.
MAMS clinical trials are a new and smarter way of testing potential treatments for progressive MS. This could deliver life-changing new treatments up to three times faster.
STOP-MS is funded by a grant from the Australian Federal Government’s Medical Research Future Fund.
You can register your interest in taking part in STOP-MS and determine if you may be eligible to participate through MS Trial Screen.
There will be multiple sites across Australia recruiting participants in:
STOP-MS uses what’s called a multi-arm, multi-stage (MAMS) design. This is similar to the design of the PLATYPUS trial, the Australian arm of the international OCTOPUS trial for MS.
MAMS trials make it possible to test new treatments up to three times faster by:
Usually, it takes around 15 years to test a potential treatment in people. A drug goes through several separate trial phases to find out whether it’s safe and effective in increasingly large numbers of people.
STOP-MS will significantly shorten that timeline, by merging 2 trials into one.
Merging separate trials may sound obvious. But launching a MAMS trial for MS needs so many things to line up perfectly. From hospitals around the country equipped to be trial sites, to the incredibly complicated statistics that underpin the design.
Your support could help us find treatments for everyone with MS.
STOP-MS is a trial which will initially test two different treatments with the ultimate aim to find one that can slow down the progression of disability in people with primary or secondary progressive MS, by suppressing the Epstein Barr virus (EBV).
Eligibility criteria can be found on the Australian New Zealand Clinical Trials Registry (ANZCTR) website.
STOP-MS aims to be a more efficient kind of clinical trial by using the multi-arm, multi-stage (MAMS) approach. It will run over a number of years. The trial has two stages.
In the first stage participants allocated to the treatment arms and placebo arm will have tests for active Epstein Barr virus (EBV) in their blood and saliva, as well as other tests. At the end of this stage, researchers will review these results.
Analysis of Stage 1 data will help researchers decide which of the treatments under investigation is more likely to be helpful in reducing levels of active EBV and ultimately slowing disease progression.
If the blood and saliva tests show that a treatment is not making a difference to EBV activity, then that arm will be stopped.
The treatment that seems to have the most beneficial effect against EBV at this point of analysis will continue to be tested in the second stage.
During the second stage more participants will be recruited to join the chosen drug treatment group from Stage 1 and the placebo group . At this stage, researchers will be looking at clinical data to see whether the treatment can, in fact, slow down the progression of MS.
You can find more information about the STOP-MS trial in this Participant Information Sheet. Please note: this document is for general information only. Please contact your nearest trial site for more information on this trial.
To learn more about MS Clinical Trials, please refer to our Trial.Smart modules available via the MS Australia website.
You can register your interest and determine if you may be eligible to participate in the STOP-MS trial via MS Trial Screen.
Other MS research
If you are a person living with MS and would like to further contribute to MS research, you can register to a survey-based research study called The Australian MS Longitudinal Study (AMSLS).
This study collects real life data from people living with MS and is used to guide policy makers, medical, and support services to create positive change and improve the lives of people with MS. For more information on this study, please refer to the MS Australia AMSLS webpage.
You can find more information about the STOP-MS trial in the Participant Information Sheet.
A lay summary of the Participant Information Sheet is also available: Lay summary
Please note: these documents are for information only. Participants going through the recruitment process will be given the Participant Information Sheet specific to their trial site.
To learn more about MS Clinical Trials in general, please refer to our Trial.Smart modules available via the MS Australia website.
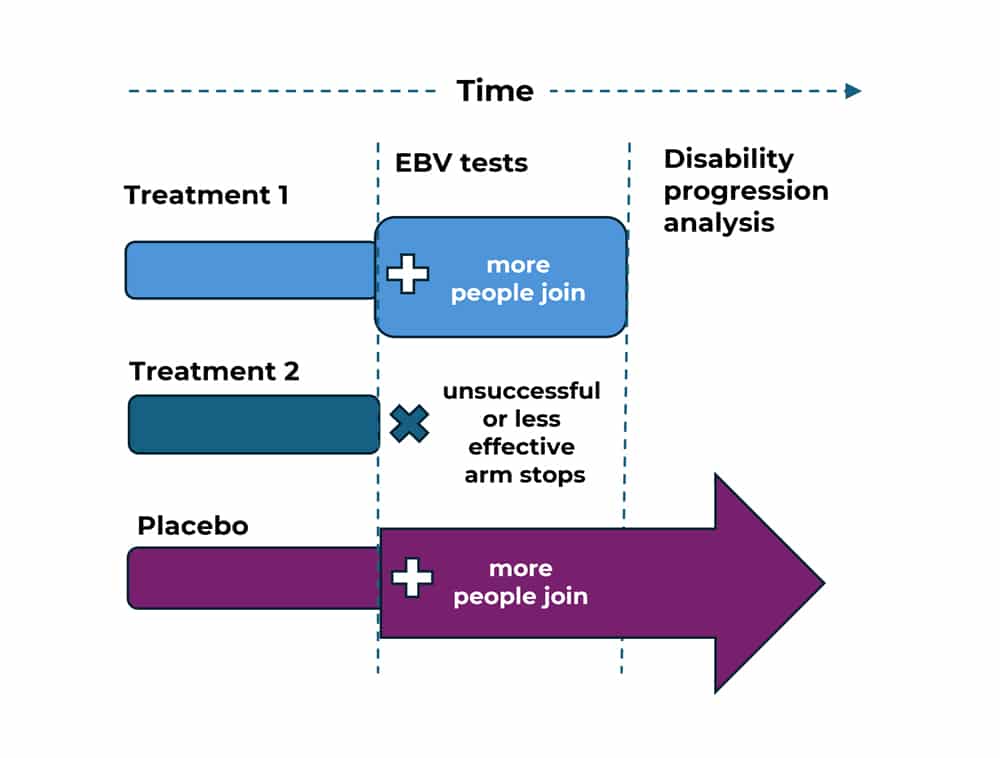
STOP-MS is using a type of trial design known as Multi-Arm Multi-Stage (MAMS).
This method can have many advantages over traditional trials:
The information on this page is adapted from the UK MS Society’s OCTOPUS trial website.