
European Medicines Agency approves alemtuzumab (Lemtrada) for relapsing remitting MS
The European Medicines Agency yesterday approved alemtuzumab (Lemtrada) for relapsing remitting MS. Alemtuzumab is an intravenous infusion drug

The European Medicines Agency yesterday approved alemtuzumab (Lemtrada) for relapsing remitting MS. Alemtuzumab is an intravenous infusion drug

Researchers have used proteomic profiling to characterise stages and severity of MS, in an attempt to provide new
Recent months have seen further publication of the findings from ongoing research into chronic cerebrospinal venous insufficiency (CCSVI)
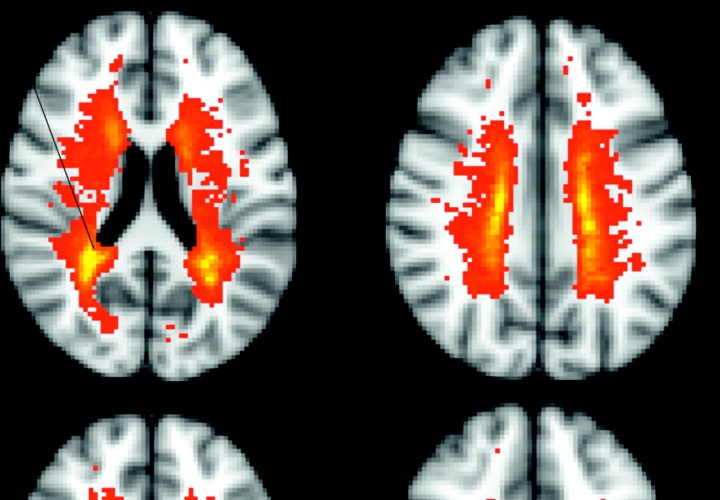
The USA Federal Drug Administration (FDA) is investigating a case of progressive multifocal leukoencephalopathy (PML) in a 46

Multiple Sclerosis Research Australia has welcomed the recent Pharmaceutical Benefits Advisory Committee (PBAC) recommendations for two new oral

ANZgene, the consortium of MS genetics researchers across Australia and New Zealand, supported by MS Research Australia, has

Dr Jeanette Lechner-Scott from the University of Newcastle talks about fatigue and cognition. They are symptoms of Multiple

An economic impact report released today details the cost of multiple sclerosis (MS) to individuals and the national

In one of the largest human genetic studies ever undertaken, scientists have identified the major common genetic variants